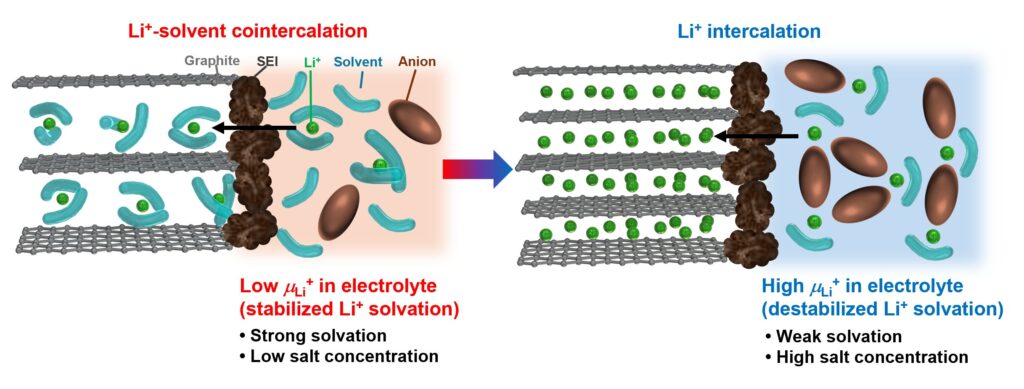
Schematic diagram of the reaction of a graphite negative electrode in different electrolytes depending on the Li+ chemical potential of the electrolyte. Credit: Advanced Materials (2025). DOI: 10.1002/adma.202514060
A joint research team from Osaka University and Daikin Industries, Ltd. has identified important new metrics for designing advanced lithium-ion batteries. They found that the electrolyte’s lithium-ion chemical potential (a measure of the “nastyness” of lithium ions within the battery’s electrolyte) quantitatively determines whether the battery can be reversibly charged and discharged.
The article “Chemical potential of electrolyte Li+ correlates with graphite anode reaction in lithium-ion batteries” is published in Advanced Materials.
This discovery paves the way from trial-and-error development to a rational, data-driven design process for safer, higher-performing batteries.
Lithium-ion batteries are essential to modern society, powering everything from smartphones to electric cars. To improve performance, researchers have been looking for new electrolytes (liquid media that transport ions). However, a major challenge is the lack of clear guidelines to predict whether new electrolytes will work well with the graphite negative electrodes commonly used in these batteries. This makes electrolyte development a difficult and empirical process.
The research team revealed that the key lies in the chemical potential of lithium ions in the electrolyte. To properly charge a battery, lithium ions must move from the electrolyte to the graphite electrode.
The researchers found that this process can only occur successfully if the lithium ions are sufficiently unstable in the electrolyte, meaning the chemical potential is high. This new metric provides clear numerical criteria for determining electrolyte compatibility and puts an end to the guesswork. Furthermore, we demonstrated that a newly developed fluorinated ether solvent designed based on this idea achieves excellent battery performance.
This discovery allows for the rational and highly efficient design of new electrolytes. By integrating the chemical potential of lithium ions into materials informatics, researchers can predict the performance of new materials and dramatically speed up the development process.
This will accelerate improvements in the performance, lifespan, and safety of batteries used in critical social infrastructure, such as electric vehicles, renewable energy storage systems, and uninterruptible power supplies for data centers.
“With this study, we not only discovered a new substance,” says Dr. Yasuyuki Kondo, lead author of the study.
“We have identified the factors that actually govern the charging and discharging reactions of lithium-ion batteries. We hope that our findings will accelerate future battery research and development and contribute to solving the world’s energy and economic challenges.”
Further information: Yasuyuki Kondo et al., Electrolyte lithium + chemical potential correlates with graphite anode reaction in lithium-ion batteries, Advanced Materials (2025). DOI: 10.1002/adma.202514060
Provided by Osaka University
Citation: New Quantitative Rules for Designing Better Batteries (November 17, 2025), Retrieved November 17, 2025 from https://techxplore.com/news/2025-11-quantitative-batteries.html
This document is subject to copyright. No part may be reproduced without written permission, except in fair dealing for personal study or research purposes. Content is provided for informational purposes only.