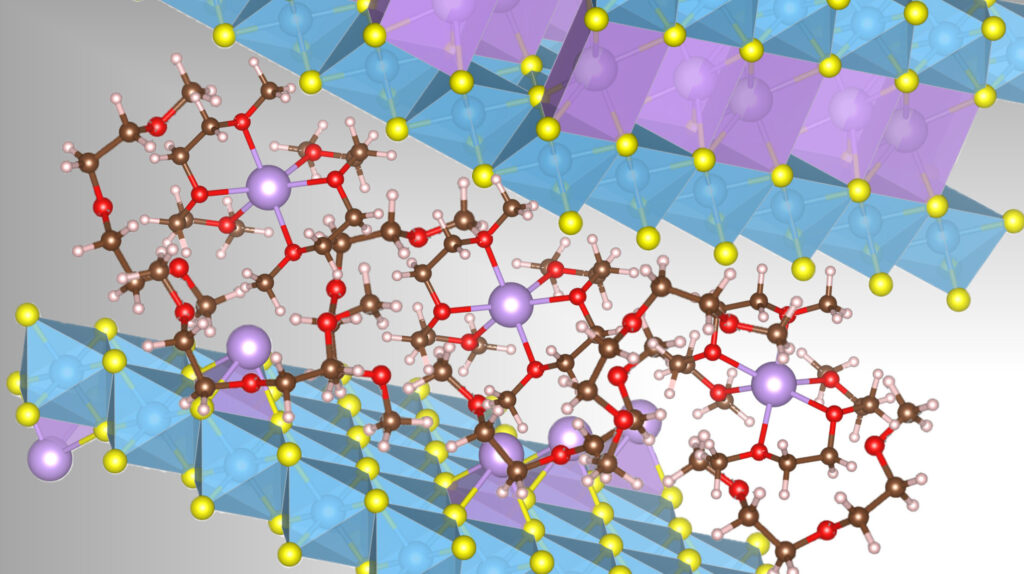
This image shows a layered structure consisting of transition metals (blue) and sulfur (yellow). The space between the layers can be occupied by sodium ions (purple) and organic solvent molecules (red, brown) through a process called co-insertion. Credit: Y. Sun et al. ,Nature Materials 2025
Li-inion and Na-ion batteries operate through a process called intercalation. Here, ions are stored and exchanged between two chemically different electrodes. In contrast, co-insertion, a process in which both ions and solvent molecules are conserved simultaneously, has traditionally been considered undesirable because it tends to cause rapid battery failure.
Contrary to this traditional view, an international research team led by Philip Adelhelm demonstrates that joint interventions can become a reversible and fast process for cathode materials in Na-Ion batteries. The approach to co-preserving ions and solvents in cathode materials provides a new handle for designing batteries with high efficiency and fast charging capabilities. The results can be found in Nature Materials.
Battery performance depends on many factors. It depends in particular on how ions are stored in the electrode material and whether they can be released again. This is because the charge carriers (ions) are relatively large and can cause undesired changes in volume as they move to the electrode. This effect, known as “breathing,” impairs the battery’s service life.
The volume change is particularly pronounced when sodium ions migrate together with molecules from the organic electrolyte. This so-called joint intervention is generally considered to be harmful to battery life. However, an international research team led by Adelhelm is currently investigating cathode materials that allow co-insertion of ions and solvent molecules, enabling faster charging and discharge processes.
Co-insertion of the anode
In previous studies, the team investigated the co-insertion of graphite anodes and demonstrated that sodium, when combined with glymm molecules, can rapidly and reversibly migrate to the electrolyte over many cycles. Nevertheless, it remained difficult to prove the same concept of cathode materials. To tackle this challenge, the team investigated a series of layered transition metal sulfides and identified the solvent co-insertion process of the cathode material.
“The collaborative intervention process can be used to develop highly efficient and fast batteries, which is why we wanted to explore this topic in greater detail,” says Professor Adelhelm.
Cathode Co-insertion: Another Process
This study incorporates detailed research from the past three years. Dr. YananSun performed volume change measurements on cathode materials and structural analysis with synchrotron radiation on Desy’s Petra III to investigate the electrochemical properties of various combinations of electrodes and solvents. Support from theory allowed us to work with Dr. Gustav Avall to identify key parameters that will help predict future co-inhalation responses.
Advantage: Ultra-fast speed
“The co-insertion process of cathode material is very different to what happens at graphite anodes,” explains Sun. Co-insertion reactions of graphite anodes usually result in low volume electrodes, but the loss of capacity caused by co-insertion of the investigated cathode material is very low.
“More than anything, certain cathode materials offer great advantages. The kinematics are very fast and almost seem to be a supercapacitor,” emphasizes Sun.
A vast chemical landscape of new materials
“The true beauty of the joint intervention response lies in its ability to provide a vast chemical landscape for designing new layered materials for a variety of applications,” Adelhelm says.
“Exploring the concept of collaborative intervention was extremely dangerous as it contradicts classical battery knowledge. The findings were the result of collaborative efforts from many talented people and were not possible without the opportunity provided by the joint research group of Operando Battery Analysis, funded by Helmholtz-Zentrum Berlin and Humboldt-University.”
“The recently announced Berlin Battery Labs between HZB, HU and BAM provide even more opportunities for the Berlin collaborative research project.”
Details: Yanan Sun et al, Solvent Co-Insertion of Layered Cathode Active Materials for Sodium-Ion Batteries, Natural Materials (2025). doi:10.1038/s41563-025-02287-7
Provided by the German Research Centre Association of Helmholtz Association
Citation: The joint intervention process enables fast-charged sodium battery (July 21, 2025) obtained from https://techxplore.com/news/2025-07-intercalation-entercalation-enables-fast-sodium-batter.html
This document is subject to copyright. Apart from fair transactions for private research or research purposes, there is no part that is reproduced without written permission. Content is provided with information only.