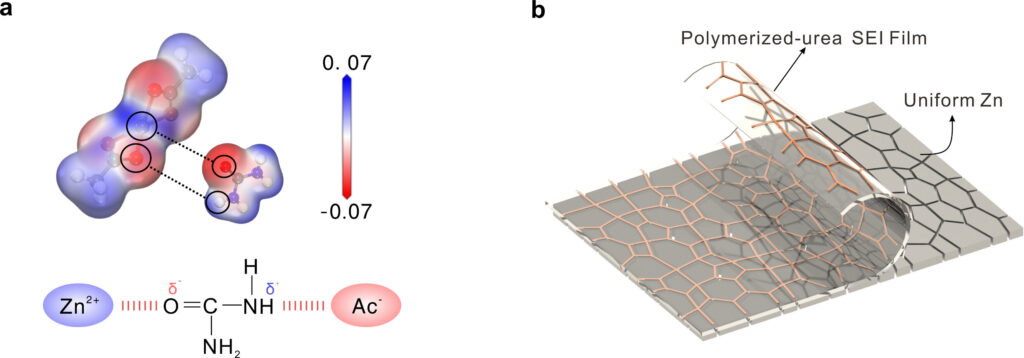
(a) Calculated electrostatic potential (ESP) distributions of Zn(AC)2 and urea, and a schematic diagram of the Zn2+-urea-Ac-Ac-system. (b) Schematic diagram of uniform Zn deposition using USPH-5. Credit: Angewandte Chemie International Edition (2025). doi:10.1002/anie.202508556
A research team led by Professor Hu Linhua of Hefei Physical Science (HFIPS) at the Chinese Academy of Sciences has developed a highly durable hydrogel electrolyte (Azibs) for aqueous zinc and ion batteries by using urea as a zinc solution and zinc acetate (Zn(AC) salt and environment for zinc solvents and zincatete).
Their findings were recently published in Angewandte Chemie International Edition.
The newly designed hydrogel can maintain tensile extension of 557% and 3.7 MPA compressive strength. The in-situ polyuurea solid electrolyte interphase (SEI) formed during Azib operations allows stable Zn stripping/plating of dendrites and allows for a non-negative method.
“This approach overcomes the usual limitations of low-cost Zn(AC)2 salts and is much better at resisting wear and tear,” said team member Li Zhaoqian. “The material can withstand the iterative process of galvanizing and stripping, as well as other physical stresses, improving overall durability.”
Aqueous Zinc-ion batteries have long faced challenges, including electrolyte leakage and electrode corrosion. Electrolytes in the semi-solid state offer better stability and flexibility, but often lack cost-effectiveness, environmental friendliness and fatigue resistance. Zinc acetate is attractive due to its low cost and environmentally friendly nature, but it has low solubility and limits battery capacity and performance.

Comparison of the characteristics of different electrolytes. Credit: Angewandte Chemie International Edition (2025). doi:10.1002/anie.202508556
To address this, researchers adopted a new strategy that utilizes the “salting” effect. This improved zinc acetate solubility by removing the hydrated layer around the polymer chains, thereby enhancing the network. This reinforcement increases fatigue resistance and allows the electrolyte to withstand repeated electrochemical cycling and external mechanical deformation.
During battery operation, a protective layer naturally forms on the electrodes, improving the overall stability of the battery’s interface. Zinc-ion batteries show excellent efficiency, and the flexible pouch cell works well in terms of capacity and stability even after many cycles. The flexibility of the pouch cell is particularly noteworthy. It is suitable for use with portable and wearable devices as it maintains a stable voltage even when bent or folded.
“When flexible pouch batteries were exposed to varying degrees of bend, they held stable voltages even at 180°. This finding highlights the potential for application in portable and wearable electronic devices,” says Dr. Li Zhaoqian.
The researchers also evaluated the battery performance in terms of rate capabilities and self-draining. The complete Zn//NH4V4O10 battery with USPH-5 electrolyte showed excellent capacity even after repeated cycling. After a long break, it still resulted in a strong discharge capacity, but the battery without USPH-5 electrolyte showed a large loss in capacity. This shows that new electrolyte materials significantly improve the overall performance of the battery and retention over time.
This study highlights the solubility of the zinc deposited state, not only disrupting the limits of salt solubility of ajib in the semi-solid state, but also provides an expandable adjustment strategy for other metal anodes to meet low-cost, environmentally friendly, high-performance batteries.
Details: Improved performance of semi-solid state zinc-ion batteries via Yifan Wang et al, Zincophilic Solubilization, Angewandte Chemie International Edition (2025). doi:10.1002/anie.202508556
Provided by the Chinese Academy of Sciences
Quote: Flexible Zinc-ion battery maintains a stable voltage after bending with a new hydrogel electrolyte (2025, June 30), obtained from https://techxplore.com/2025-06 on June 30, 2025.
This document is subject to copyright. Apart from fair transactions for private research or research purposes, there is no part that is reproduced without written permission. Content is provided with information only.