Credits: Resources, Conservation, Recycling (2024). doi:10.1016/j.resconRec.2024.107980
A team of researchers at UCONN College of Engineering (COE), led by Burcu Beykal, an assistant professor at COE’s Department of Chemicals and Biomolecular Engineering, is studying how lithium can be extracted from a variety of sources, including salt and seawater. Lithium has become a very, very popular resource in the field of energy storage and sustainability, especially with the surge in everyday electronics such as electric vehicles and mobile phones and portable computers.
According to Beykal, many important sectors (such as energy, defense, and high-tech manufacturing) rely on the availability of non-substituted resources known as key minerals. Lithium is at the top of that list. It is an important functional material for the cathodes and electrolytes of lithium-ion batteries, and is therefore the elemental backbone of modern energy storage systems.
Furthermore, nearly a third of US greenhouse gas emissions, mainly from carbon dioxide (CO2), comes from the transportation sector, where around 200 billion gallons of gasoline are consumed each year. Also, lithium demand will skyrocket if global electric vehicle market forecasts are projected to increase by 10 times by 2030.
“Most lithium extraction occurs in only a few regions worldwide, creating risks to the US supply chain,” says Baikal. “My research explores ways to restore lithium more sustainable and domestically, particularly from unutilized sources such as geothermal salt water found in the US, “Can we design a better process for economically and environmentally domestic lithium recovery?”
According to a report in the US Geological Survey (USGS) 2023, approximately 80% of the global lithium supply is allocated to battery use, reflecting the important role of lithium in lithium-ion batteries and the important demand in a variety of industries, including light aviation alloys, glass production, catalyst manufacturers and the space industry.
“Without an environmentally sustainable and affordable solution, demand is at a turning point where lithium supply availability is far beyond its availability,” says Beykal. “The ocean contains a vast reserve of approximately 231.4 trillion tons of dissolved lithium, but their low concentrations do not economically survive lithium recovery from seawater. Therefore, lithium is currently only mined from salt lakes and continental saltwater.
Lithium demand exceeds supply
Approximately 98 million tonnes of lithium resources are available worldwide based on the recent USGS report (US Geological Survey, 2023). However, only 26 tons of extraction can be accessed. Additionally, environmental concerns about the processing of ore and salt lake saltwater, and access to traditional ingredients around the world, have led to increased interest in the United States in extracting lithium from unconventional sources, such as geothermal salt water discovered along the West Coast of California.
According to Beykal, geothermal salt water is a new resource, providing both renewable energy and essential minerals from a single operation. These hot saline from underground on Earth generate electricity by turning the turbine when pumped onto the surface. It is also rich in minerals as hot fluids circulating through the mineral-containing rock formations dissolve minerals and metals in the solution.
Large quantities of lithium, along with other valuable minerals such as boron and potassium, make geothermal salt water the desirable raw material for lithium recovery. Therefore, as of November 2023, the US Department of Energy recognized California’s Salton Sea region as a domestic lithium resource.
The challenges go beyond geographical limits, Baikal notes. Additionally, current extraction methods are environmentally unsustainable, available resources are not easily accessible, extraction requires substantial amounts of water and energy, and high chemical, residual and airborne emissions.
Additionally, there is a lack of detailed process modeling and economic and environmental assessments of large-scale lithium recovery processes from unconventional sources,” says Baikal. “Integrating technoeconomy assessment and lifecycle analysis into the assessment of the lithium recovery process is important to understand the feasibility of the economy and its impact on the environment.”
Beykal and her team also include Hasan Nikkhah, Professor Andrea Di Maria of De Liège University and Professor Giuseppe Granata of Ku Leuven. Their work incorporates on-site recycling technology to effectively reduce operational costs and reduce innovative steps to effectively reduce carbon footprint by 50%.
Nikka is a graduate student who works with Beikal. His research role focuses on the technical and economic aspects of lithium recovery from geothermal waters. In particular, they say there is room for immediate improvement in order to integrate the lithium recovery process with carbon capture technology to make the process more sustainable and versatile.
“After developing the process model, we expanded our scope to determine how the US could build a resilient domestic EBOP supply chain,” explains Nikkhah. “The next step is to develop mathematical models to design and optimize the supply chain, and place lithium extraction, battery manufacturing and EV production plants across the state to identify where they need to meet the EV demands of each state.
Improved processes and reduced environmental impact
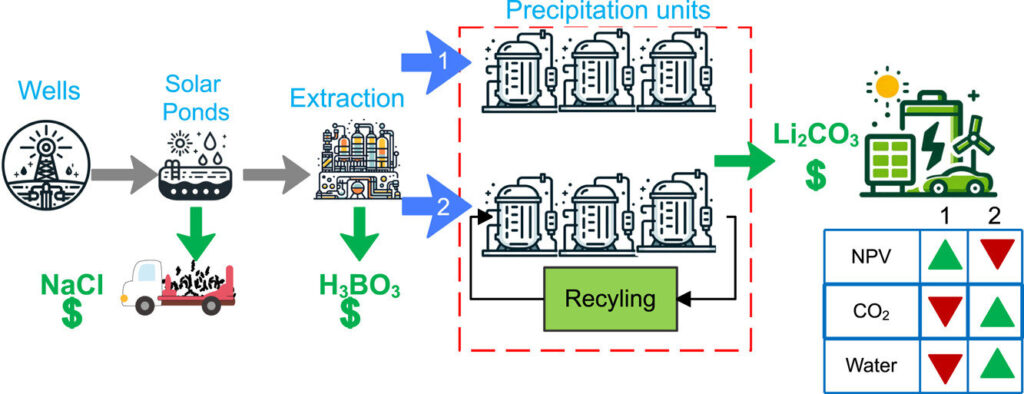
The team also proposed a new recycling process for on-site reuse of precipitate agents. This has reduced CO2 emissions by almost half. This study has been published in Resources, Conservation and Recycling.
“Our research is trying to develop the first end-to-end digital twin of a large-scale sustainable lithium production process from geothermal salts using chemical precipitation,” explains Beykal. “Lithium extraction research is certainly attracting attention and we are at the forefront of this initiative. As a leader in this strategically important field, we are building the unique expertise of large-scale lithium recovery and positioning UConn and the Engineering University.”
Baikal and her team now map the wider US lithium supply chain and understand how a variety of domestic ingredients contribute, including geothermal salt water, seawater, clay and recycled batteries. We also extend our computational models to assess and optimize the scope of our extraction strategies, as well as those we have studied so far.
Through this work, Nikka says he has gained a deeper appreciation for how complex and interconnected clean energy systems are, and talks about how valuable their work is for environmental sustainability and job creation.
“All decisions, such as where to place the facility, the extent to which materials are transported, have ripple effects on the overall efficiency, cost and environmental impact of the supply chain,” emphasizes Nikka.
“Lithium is not a critical component of clean energy, but a strategic resource related to national security and economic resilience. Through our research, we have learned that domestic lithium supply chains can generate more than 100,000 direct jobs in the United States.
Details: Hasan Nikkhah et al., Sustainable Process Design for Lithium Recovery from Geothermal Saline Using Chemical Precipitation, Resources, Conservation and Recycling (2025). doi:10.1016/j.resconRec.2024.107980
Provided by the University of Connecticut
Quote: Geothermal Salt may retain the key to maintaining energy challenges, obtained on July 15, 2025 from https://techxplore.com/news/2025-07-geothermal-brine-key-key-eenergy.html (July 15, 2025)
This document is subject to copyright. Apart from fair transactions for private research or research purposes, there is no part that is reproduced without written permission. Content is provided with information only.