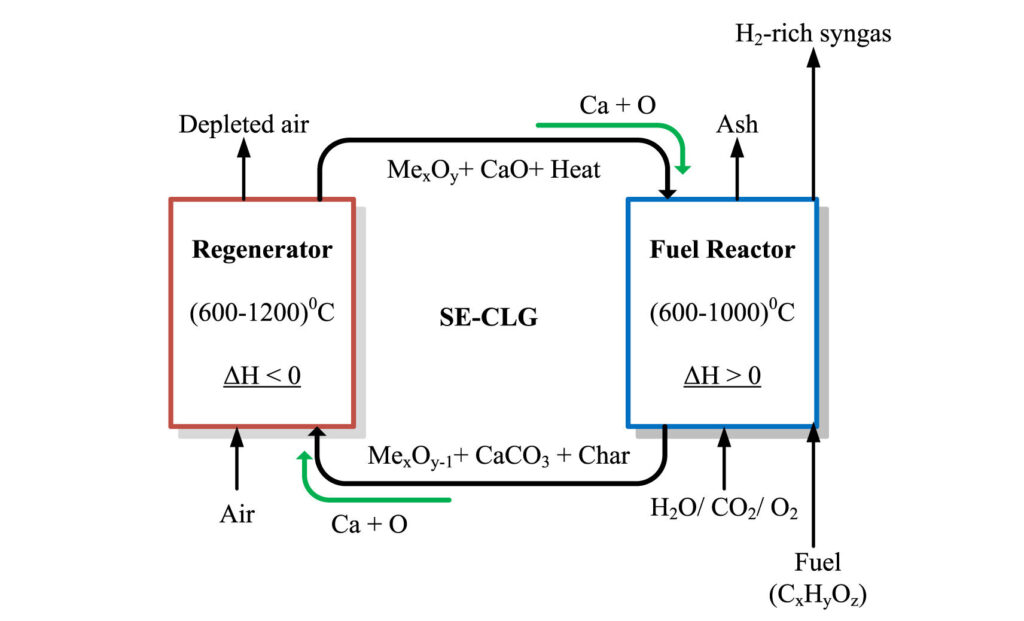
Redox-based design of the experimental approach of SECLG of fuel residues. Credit: Renewable Energy (2025). doi: 10.1016/j.renene.2025.123022
A promising industrial process can convert grinded sugarcane waste into green hydrogen much more efficiently than previously thought, showing the SECLG process simulation at the University of Johannesburg. This research is published in Renewable Energy.
This simulation shows high energy efficiency and produces a small portion of unwanted tar, carbon monoxide (CO), carbon dioxide (CO2), and nitrogen (N) compared to traditional biomass gasification plants. This process could help decarbonize energy-intensive industries such as steel and cement in the future.
Sugarcane and power grid
Approximately 1.4 billion tons of sugar cane are produced worldwide every year. Then, about 540 million tons of grinded sugarcane waste biomass (known as bagasse) is produced. Countries such as India, China, Brazil and Mauritius have already gasified bagasse and powered the national power grid.
Gasification is a method of “chemically burning” biomass and converting it into syngas, a clean mixture of hydrogen and other gases. However, there are no traditional fires.
Too much tar
The large-scale gasification methods currently in use are not energy efficient, produce high-speed hydrogen, and produce tar and other harmful by-products, says Professor Bilainu Oboirien of the University of Johannesburg. He is a researcher in the Department of Chemical Engineering and Technology.
“The typical Singapore Singa for biomass gasification has a balance of hydrogen (10-35%), carbon monoxide (20-30%), carbon dioxide (10-25%), TAR (10-100 g/nm3), nitrogen (40-50%) and hydrocarbons,” says Oboirien.
“Here, the carbon dioxide produced is not captured by the process. Also, high tar yields require a lot of additional equipment for cleaning. In the context, tar is like dirty engine oil in a car. This greatly increases the operating costs,” he adds.
A better way to green hydrogen
A much more effective method for gasifying biomass such as bagasse is called chemical loop gasification (SECLG), which enhances sorption. Various research groups have been developing SECLG over the past decade.
Compared to the methods used in today’s industry, SECLG can produce green hydrogen with a much higher purity with a higher yield from biomass. It is also much more energy efficient and allows better capture of carbon within the process itself, Oboirien says.
Low tar process simulation
Professor Oboirien and UJ Master candidate Lebohang Gerald Motsoeneng have created a mathematical model of the SECLG process.
They followed this with a comprehensive Aspen Plus simulation of the SECLG process on a laboratory scale. They compared two known metal oxides used as oxygen carriers in the process to see how these affect hydrogen yield and other parameters.
Higher hydrogen yield
“For SECLG, our model estimates the balance of hydrogen (62-69%), carbon monoxide (5-10%), carbon dioxide (less than 1%), TAR (less than 1 g/nm3), nitrogen (less than 5%) and hydrocarbons,” Oboirien said.
This means that high hydrogen yields, low tar concentrations, and low nitrogen dilution can significantly reduce economic costs by reducing the number of additional equipment required.
The quality of the hydrogen is expected to be good. However, he adds that further cleanup will be required to reach industrial grade gases that are readily usable for linked processes.
Existing infrastructure
Countries with immediate access to existing biomass gasification infrastructure and biomass can benefit most from Bagasse’s SECLG for green hydrogen, Oboirien says. Examples are China, Brazil and South Africa. This is because it’s much easier and cheaper to modify existing technology than to acquire and build a new, dedicated SECLG plant, he says.
Tuning with an oxygen carrier
The Aspen Plus model compares the efficiencies of high-performance oxygen carriers, well-known metal oxides nickel oxide (NIO) and iron oxide (Fe2O3). The study also examines the stability of oxygen carriers and adsorbent materials, taking into account the harsh conditions during SECLG caused by high temperatures, pressures, and material transport systems, Oboirien says.
This model shows that oxygen carrier nickel oxide produces hydrogen with higher purity, and more effectively captures carbon dioxide in the reactor during the process.
On the other hand, iron oxide, another oxygen carrier, is excellent at producing more flammable gas blends. It also identifies the possibility of an adjustable SECLG process that produces transport fuels such as diesel in addition to hydrogen.
Next Steps
Currently, this model does not address the degradation of oxygen carriers and adsorbent materials over time in real-world applications. Furthermore, the transmission and efficient separation of unwanted ash and char solid materials were not modeled or simulated, but these are required for viable SECLG systems.
Oboirien said, “We are currently developing experimental further proofs of the concept in a laboratory-scale environment. We hope that through these experiments we can validate these models against experimental data.”
Scale up
SECLG is a proven concept using process simulation models, but it has its own unique challenges. It is not yet used in syngas operations from large industrial biofuels.
Oboirien says SECLG requires a temperature of about 600 degrees Celsius, a pressure of about 5 bars, and multiple cycles. Seglg also requires a transport system for metal oxide oxygen factors and adsorbent materials in this case. These allow for continuous catalysts and carbon capture cycles “loop effects” of the process.
“Adsorption-enhanced chemical loop gasification biomass is a promising process for generating hydrogen and transport fuels,” Oboirien said.
“This study requires infrastructure investments and inter-industrial collaboration to be sustainable, and hopefully realize the possibilities of this SECLG technology,” he adds.
Details: Lebohang Gerald Motsoeneng et al, H2 and transport fuel production, adsorption-enhanced chemical loop gasification of biomass of renewable energy (2025). doi: 10.1016/j.renene.2025.123022
Provided by the University of Johannesburg
Quote: High purity green hydrogen with very low TAR from biomass, retrieved from July 14, 2025 with chemical loop gasification (2025, July 14) https://techxplore.com/news/2025-07-high-purity-green-hydrogen-tar.html
This document is subject to copyright. Apart from fair transactions for private research or research purposes, there is no part that is reproduced without written permission. Content is provided with information only.