The β-namno2 stacking fault significantly reduces capacity during the charging/emission cycle. Copper doping effectively eliminates stacking obstacles, greatly improving cycling stability, and enables long-term development of sodium ion batteries. Credit: Professor Komaba, Tokyo University of Science, Japan
Sodium (NA)-ion batteries have recently emerged as a cost-effective and sustainable alternative to lithium (LI)ion batteries. The sixth most abundant element on the planet, Na offers lower material costs and availability compared to Li-ion batteries.
The design of the cathode material plays a key role in determining battery life and stability. Layered sodium manganese oxide (NAMNO2) has attracted attention from researchers for its use as a cathode material for Na-Ion batteries.
NAMNO2 exists in two crystal forms, α-namno2 and β-namno2. The α-phase features a monocular layered structure in which a planar MnO2 layer consisting of edge-covalently distorted MnO6 octahedra stacked alternately with the Na-ions between them.
β-namno2, on the other hand, features a distorted Mno6 octahedra cardboard or zigzag layer with edge sharing, using naion during that time. Higher temperatures are usually required for the synthesis of β-namno2, which often leads to the Na deficiency stage.
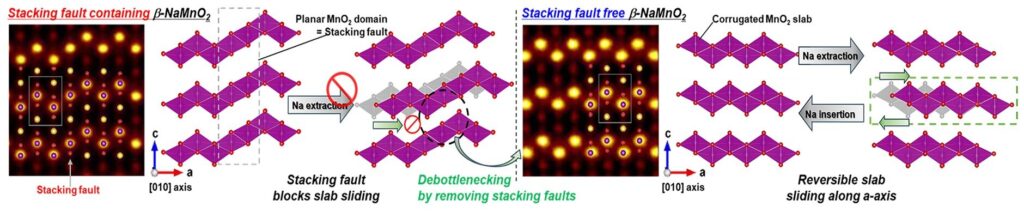
An attempt to prevent NA-deficient phases produces a non-equilibrium β-phase that exhibits several defects. Most notable of these are stacking faults (SFS), formed by sliding in the crystallographic BC plane and produced by generating stacking sequences similar to the α-phase.
Electrodes made from β-NAMNO2 containing SF suffer from severe capacity reduction during the charging/emission cycle, limiting practical applications. Furthermore, SFS complicates understanding of solid-state chemistry of materials.
In the new study, a research team led by Professor Komabajima of the Department of Applied Chemistry at the University of Tokyo Science (TUS) in Japan investigated how copper (CU) doping stabilizes the SFS of β-NAMNO2.
“Previous studies have found that among metal dopants, Cu is the only dopant that can successfully stabilize β-Namno2,” explains Professor Komaba.
“In this study, we systematically investigated how Cu doping suppresses SF and improves the electrochemical performance of β-NAMNO2 electrodes in Na-Ion batteries.”
The team also included Syuhei Sato, Mira Yusuke Yusuke Mira and Dr. kumakura from the Institute of Science and Technology at Tus. Their findings were published online in Journal Advanced Materials.
The team synthesized highly crystalline Cu-doped β-Namno2 samples (NAMN1-XCUXO2) using different amounts of Cu, presented as NMCO -00, -05, -10, -12, and -15.
The NMCO-00 samples served as non-gaze references. X-ray diffraction (XRD) studies have found that NMCO-05 exhibits the highest SF concentration at 4.4% among Cu-doped samples, while NMCO-12 has a SF concentration of only 0.3%, indicating a clear suppression of SFS with increased Cu doping.
Electrochemical evaluation of electrodes made from NA semi-cell NMCO samples revealed a significant enhancement of the capacity retention of Cu-doped samples. The non-gaze samples showed rapid capacity loss within 30 cycles, whereas the SF-free NMCO-12 and -15 samples showed excellent cycle stability, while the NMCO-12 showed no capacity loss over 150 cycles.
These results suggest that the β phase of layered nAMNO2 is inherently stable when SF is eliminated.
Importantly, the SF-free structure allowed researchers to examine the complex phase transitions that occur during NA insertion and extraction of these materials.
Using a combination of in situ and ex situ XRD measurements and sensory theoretical calculations of density, the researchers proposed a new structural model that includes dramatic gliding of the corrugated MnO2 layer.
This gliding appears to be intrinsic to the β phase and was previously obscure by the presence of SFS, indicating a major advance in understanding the characteristic structural changes in the β phase of nAMNO2 during the electrode reaction.
“Our findings confirm that manganese-based oxides are promising and sustainable solutions for developing durable Na-Ion batteries,” says Professor Komaba.
“The relatively low cost of manganese and NA leads to more affordable energy storage solutions for a variety of applications, including smartphones and electric vehicles, and ultimately leads to a more sustainable future.”
The study also shows that stabilization of SF using Cu doping can resolve supply chain vulnerabilities commonly faced with metals such as lithium. Furthermore, this study has potential implications for grid storage, electric vehicles, and home appliances.
This study provides valuable insights for developing more stable and long-term Na-ion batteries, leading to wider adoption of renewable energy, and collaborates with the United Nations Sustainable Development Goal 7: Affordable Clean Energy.
Details: kumakura et al et al, unobstructed β-munamno2, synthesis and electrochemistry of advanced materials (2025). doi:10.1002/adma.202507011
Provided by Tokyo University of Science
Quote: Scientists Discover Keys for Stable, High Performance, and Long-Term Sodium-Ion Battery (July 16, 2025) From July 16, 2025 https://techxplore.com/news/2025-07-scientists-uncover-key-table-htmll
This document is subject to copyright. Apart from fair transactions for private research or research purposes, there is no part that is reproduced without written permission. Content is provided with information only.